Coeliac disease is a common enteropathy that occurs in genetically susceptible individuals in response to the ingestion of gluten proteins present in wheat, rye and barley. Currently, the only available treatment for the condition is a strict, life-long gluten-free diet that, despite being safe and often effective, is associated with several challenges. Due to the high cost, particularly restrictive nature and perception of decreased quality of life associated with the diet, some patients are continuously exposed to gluten, which prevents an adequate disease control. Moreover, a subgroup of patients does not respond to the diet adequately, and healing of the small-bowel mucosa can be incomplete. Thus, there is a need for alternative treatment forms. The increasingly understood pathogenetic process of coeliac disease has enabled the identification of various targets for future therapies. Multiple investigational therapies ranging from tolerogenic to immunological approaches are in the pipeline, and several drug candidates have entered phase II/III clinical trials. This Review gives a broad overview of the different investigative treatment modalities for coeliac disease and summarizes the latest advances in this field.
Key points
- At present, a gluten-free diet is the only effective treatment for coeliac disease but is associated with several possible challenges, including a high economic and societal burden, inferior quality of life and sometimes inadequate response.
- An increased understanding of the pathogenetic process in coeliac disease has revealed various therapeutic targets for future drugs that could complement or replace a gluten-free diet.
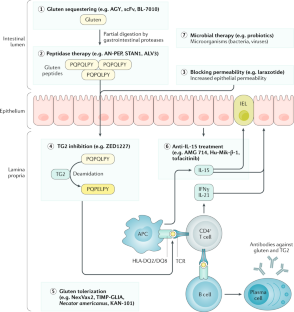
- Novel therapeutic strategies include approaches to detoxify gluten already in the gastrointestinal tract by sequestrants or peptidases.
- Other investigational approaches comprise blocking intestinal epithelial permeability or the enzymatic activity of transglutaminase 2.
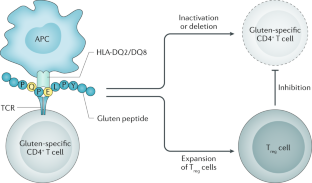
- Restoring immune tolerance to gluten or targeting the gluten-induced immune activation has also been investigated as possible therapeutic options.
- The most advanced drug candidates have now entered phase III clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
206,07 € per year
only 17,17 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
The global burden of coeliac disease: opportunities and challenges
Article 03 January 2022

Guidelines for best practices in monitoring established coeliac disease in adult patients
Article 18 December 2023
Society for the Study of Celiac Disease position statement on gaps and opportunities in coeliac disease
Article 15 September 2021
References
- Singh, P. et al. Global prevalence of celiac disease: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol.16, 823–836.e2 (2018). PubMedGoogle Scholar
- Ludvigsson, J. F. et al. The Oslo definitions for coeliac disease and related terms. Gut62, 43–52 (2013). PubMedGoogle Scholar
- Tye-Din, J. A., Galipeau, H. J. & Agardh, D. Celiac disease: a review of current concepts in pathogenesis, prevention, and novel therapies. Front. Pediatr.6, 350 (2018). PubMedPubMed CentralGoogle Scholar
- Al-Toma, A. et al. European society for the study of coeliac disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol. J.7, 583–613 (2019). PubMedPubMed CentralGoogle Scholar
- Husby, S., Murray, J. A. & Katzka, D. A. AGA clinical practice update on diagnosis and monitoring of celiac disease - changing utility of serology and histologic measures: expert review. Gastroenterology156, 885–889 (2019). PubMedGoogle Scholar
- Baggus, E. M. R. et al. How to manage adult coeliac disease: perspective from the NHS England rare diseases collaborative network for non-responsive and refractory coeliac disease. Frontline Gastroenterol.11, 235–242 (2019). PubMedPubMed CentralGoogle Scholar
- Hall, N. J., Rubin, G. & Charnock, A. Systematic review: adherence to a gluten-free diet in adult patients with coeliac disease. Aliment. Pharmacol. Ther.30, 315–330 (2009). CASPubMedGoogle Scholar
- Weisbrod, V. M. et al. A quantitative assessment of gluten cross-contact in the school environment for children with celiac disease. J. Pediatr. Gastroenterol. Nutr.70, 289–294 (2020). PubMedPubMed CentralGoogle Scholar
- Lee, A., Wolf, R., Lebwohl, B., Ciaccio, E. & Green, P. Persistent economic burden of the gluten free diet. Nutrients11, 399 (2019). PubMed CentralGoogle Scholar
- Shah, S. et al. Patient perception of treatment burden is high in celiac disease compared with other common conditions. Am. J. Gastroenterol.109, 1304–1311 (2014). PubMedPubMed CentralGoogle Scholar
- Daveson, A. J. M. et al. Baseline quantitative histology in therapeutics trials reveals villus atrophy in most patients with coeliac disease who appear well controlled on gluten-free diet. GastroHep2, 22–30 (2020). Google Scholar
- Lebwohl, B. et al. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann. Intern. Med.159, 169–175 (2013). PubMedPubMed CentralGoogle Scholar
- Shan, L. et al. Structural basis for gluten intolerance in celiac sprue. Science297, 2275–2279 (2002). CASPubMedGoogle Scholar
- Dieterich, W. et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med.3, 797–801 (1997). CASPubMedGoogle Scholar
- Cardoso-Silva, D. et al. Intestinal barrier function in gluten-related disorders. Nutrients11, 2325 (2019). CASPubMed CentralGoogle Scholar
- Moreno, M. et al. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut66, 250–257 (2017). CASPubMedGoogle Scholar
- Iversen, R. et al. Evidence that pathogenic transglutaminase 2 in celiac disease derives from enterocytes. Gastroenterologyhttps://doi.org/10.1053/j.gastro.2020.04.018 (2020). ArticlePubMedGoogle Scholar
- Molberg, O. et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med.4, 713–717 (1998). CASPubMedGoogle Scholar
- Kuja-Halkola, R. et al. Heritability of non-HLA genetics in coeliac disease: a population-based study in 107 000 twins. Gut65, 1793–1798 (2016). PubMedGoogle Scholar
- Sollid, L. M. et al. Update 2020: nomenclature and listing of celiac disease-relevant gluten epitopes recognized by CD4 + T cells. Immunogenetics72, 85–88 (2020). PubMedGoogle Scholar
- Hardy, M. Y. et al. Characterisation of clinical and immune reactivity to barley and rye ingestion in children with coeliac disease. Gut69, 830–840 (2020). CASPubMedGoogle Scholar
- Tye-Din, J. A. et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci. Transl Med.2, 41ra51 (2010). PubMedGoogle Scholar
- du Pré, M. F. et al. B cell tolerance and antibody production to the celiac disease autoantigen transglutaminase 2. J. Exp. Med.217, e20190860 (2020). PubMedGoogle Scholar
- Di Sabatino, A. et al. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut55, 469–477 (2006). PubMedPubMed CentralGoogle Scholar
- Abadie, V. et al. IL-15, gluten and HLA-DQ8 drive tissue destruction in coeliac disease. Nature578, 600–604 (2020). CASPubMedPubMed CentralGoogle Scholar
- Bouziat, R. et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science356, 44–50 (2017). CASPubMedPubMed CentralGoogle Scholar
- Caminero, A. et al. Duodenal bacterial proteolytic activity determines sensitivity to dietary antigen through protease-activated receptor-2. Nat. Commun.10, 1198 (2019). PubMedPubMed CentralGoogle Scholar
- Kemppainen, K. M. et al. Factors that increase risk of celiac disease autoimmunity after a gastrointestinal infection in early life. Clin. Gastroenterol. Hepatol.15, 694–702.e5 (2017). PubMedGoogle Scholar
- Lindfors, K. et al. Metagenomics of the faecal virome indicate a cumulative effect of enterovirus and gluten amount on the risk of coeliac disease autoimmunity in genetically at risk children: the TEDDY study. Gut69, 1416–1422 (2019). PubMedGoogle Scholar
- Kahrs, C. R. et al. Enterovirus as trigger of coeliac disease: nested case-control study within prospective birth cohort. BMJ364, l231 (2019). PubMedPubMed CentralGoogle Scholar
- Caminero, A., Meisel, M., Jabri, B. & Verdu, E. F. Mechanisms by which gut microorganisms influence food sensitivities. Nat. Rev. Gastroenterol. Hepatol.16, 7–18 (2019). CASPubMedPubMed CentralGoogle Scholar
- Kasarda, D. D. Can an increase in celiac disease be attributed to an increase in the gluten content of wheat as a consequence of wheat breeding? J. Agric. Food Chem.61, 1155–1159 (2013). CASPubMedPubMed CentralGoogle Scholar
- García-Molina, M., Giménez, M., Sánchez-León, S. & Barro, F. Gluten free wheat: are we there? Nutrients11, 487 (2019). PubMed CentralGoogle Scholar
- Hujoel, I. A. & Murray, J. A. Refractory celiac disease. Curr. Gastroenterol. Rep.22, 18 (2020). PubMedGoogle Scholar
- Silvester, J. A. et al. Most patients with celiac disease on gluten-free diets consume measurable amounts of gluten. Gastroenterology158, 1497–1499.e1 (2019). PubMedGoogle Scholar
- Lerner, B. A. et al. Detection of gluten in gluten-free labeled restaurant food: analysis of crowd-sourced data. Am. J. Gastroenterol.114, 792–797 (2019). PubMedPubMed CentralGoogle Scholar
- Viitasalo, L. et al. Microbial biomarkers in patients with nonresponsive celiac disease. Dig. Dis. Sci.63, 3434–3441 (2018). CASPubMedGoogle Scholar
- Garber, M. E. et al. A B-cell gene signature correlates with the extent of gluten-induced intestinal injury in celiac disease. Cell. Mol. Gastroenterol. Hepatol.4, 1–17 (2017). PubMedPubMed CentralGoogle Scholar
- Catassi, C. et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am. J. Clin. Nutr.85, 160–166 (2007). CASPubMedGoogle Scholar
- Lähdeaho, M.-L., Mäki, M., Laurila, K., Huhtala, H. & Kaukinen, K. Small-bowel mucosal changes and antibody responses after low- and moderate-dose gluten challenge in celiac disease. BMC Gastroenterol.11, 129 (2011). PubMedPubMed CentralGoogle Scholar
- Thompson, T., Dennis, M., Higgins, L. A., Lee, A. R. & Sharrett, M. K. Gluten-free diet survey: are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J. Hum. Nutr. Diet.18, 163–169 (2005). CASPubMedGoogle Scholar
- Di Nardo, G. et al. Nutritional deficiencies in children with celiac disease resulting from a gluten-free diet: a systematic review. Nutrients11, 1588 (2019). PubMed CentralGoogle Scholar
- Raehsler, S. L., Choung, R. S., Marietta, E. V. & Murray, J. A. Accumulation of heavy metals in people on a gluten-free diet. Clin. Gastroenterol. Hepatol.16, 244–251 (2018). CASPubMedGoogle Scholar
- Kabbani, T. A. et al. Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Aliment. Pharmacol. Ther.35, 723–729 (2012). CASPubMedGoogle Scholar
- Singh, J. & Whelan, K. Limited availability and higher cost of gluten-free foods. J. Hum. Nutr. Diet.24, 479–486 (2011). CASPubMedGoogle Scholar
- Mårild, K. et al. Celiac disease and anorexia nervosa: a nationwide study. Pediatrics139, e20164367 (2017). PubMedGoogle Scholar
- Silvester, J. A., Weiten, D., Graff, L. A., Walker, J. R. & Duerksen, D. R. Living gluten-free: adherence, knowledge, lifestyle adaptations and feelings towards a gluten-free diet. J. Hum. Nutr. Diet.29, 374–382 (2016). CASPubMedGoogle Scholar
- Zingone, F. et al. Psychological morbidity of celiac disease: a review of the literature. U Eur. Gastroenterol. J.3, 136–145 (2015). Google Scholar
- König, J., Holster, S., Bruins, M. J. & Brummer, R. J. Randomized clinical trial: Effective gluten degradation by Aspergillus niger-derived enzyme in a complex meal setting. Sci. Rep.7, 13100 (2017). PubMedPubMed CentralGoogle Scholar
- Daveson, A. J. et al. Effect of hookworm infection on wheat challenge in celiac disease–a randomised double-blinded placebo controlled trial. PLoS ONE6, e17366 (2011). CASPubMedPubMed CentralGoogle Scholar
- Mansikkka, E. et al. Gluten challenge induces skin and small-bowel relapse in long-term gluten-free diet -treated dermatitis herpetiformis. J. Invest. Dermatol.139, 2108–2114 (2019). Google Scholar
- Daveson, A. J. M. et al. Masked bolus gluten challenge low in FODMAPs implicates nausea and vomiting as key symptoms associated with immune activation in treated coeliac disease. Aliment. Pharmacol. Ther.51, 244–252 (2020). CASPubMedGoogle Scholar
- Ludvigsson, J. F. et al. Outcome measures in coeliac disease trials: the Tampere recommendations. Gut67, 1410–1424 (2018). CASPubMedGoogle Scholar
- McCambridge, J., Witton, J. & Elbourne, D. R. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J. Clin. Epidemiol.67, 267–277 (2014). PubMedPubMed CentralGoogle Scholar
- Gopalakrishnan, S. et al. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides35, 86–94 (2012). CASPubMedGoogle Scholar
- Paterson, B. M., Lammers, K. M., Arrieta, M. C., Fasano, A. & Meddings, J. B. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment. Pharmacol. Ther.26, 757–766 (2007). CASPubMedGoogle Scholar
- Leffler, D. A. et al. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am. J. Gastroenterol.107, 1554–1562 (2012). CASPubMedPubMed CentralGoogle Scholar
- Kelly, C. P. et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment. Pharmacol. Ther.37, 252–262 (2013). CASPubMedGoogle Scholar
- Leffler, D. A. et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology148, 1311–9.e6 (2015). CASPubMedGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03569007 (2019).
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT00620451 (2017).
- Cavaletti, L. et al. E40, a novel microbial protease efficiently detoxifying gluten proteins, for the dietary management of gluten intolerance. Sci. Rep.9, 13147 (2019). PubMedPubMed CentralGoogle Scholar
- Rey, M. et al. Addressing proteolytic efficiency in enzymatic degradation therapy for celiac disease. Sci. Rep.6, 30980 (2016). CASPubMedPubMed CentralGoogle Scholar
- Tye-Din, J. A. et al. The effects of ALV003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clin. Immunol.134, 289–295 (2010). CASPubMedGoogle Scholar
- Wolf, C. et al. Engineering of Kuma030: a gliadin peptidase that rapidly degrades immunogenic gliadin peptides in gastric conditions. J. Am. Chem. Soc.137, 13106–13113 (2015). CASPubMedPubMed CentralGoogle Scholar
- Bethune, M. T. & Khosla, C. Oral enzyme therapy for celiac sprue. Methods Enzymol.502, 241–271 (2012). CASPubMedPubMed CentralGoogle Scholar
- Mitea, C. et al. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut57, 25–32 (2008). CASPubMedGoogle Scholar
- Tack, G. J. et al. Consumption of gluten with gluten-degrading enzyme by celiac patients: a pilot-study. World J. Gastroenterol.19, 5837–5847 (2013). CASPubMedPubMed CentralGoogle Scholar
- Salden, B. N. et al. Randomised clinical study: Aspergillus niger-derived enzyme digests gluten in the stomach of healthy volunteers. Aliment. Pharmacol. Ther.42, 273–285 (2015). CASPubMedPubMed CentralGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT00810654 (2011).
- Ehren, J. et al. A food-grade enzyme preparation with modest gluten detoxification properties. PLoS ONE4, e6313 (2009). PubMedPubMed CentralGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT00962182 (2018).
- Korponay-Szabó, I. R. et al. Food-grade gluten degrading enzymes to treat dietary transgressions in coeliac adolescents. J. Pediatr. Gastroenterol. Nutr.50, E68 (2010). Google Scholar
- Gass, J., Bethune, M. T., Siegel, M., Spencer, A. & Khosla, C. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology133, 472–480 (2007). CASPubMedGoogle Scholar
- Siegel, M. et al. Safety, tolerability, and activity of ALV003: results from two phase 1 single, escalating-dose clinical trials. Dig. Dis. Sci.57, 440–450 (2012). CASPubMedGoogle Scholar
- Lähdeaho, M.-L. et al. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology146, 1649–1658 (2014). PubMedGoogle Scholar
- Murray, J. et al. No difference between latiglutenase and placebo in reducing villous atrophy or improving symptoms in patients with symptomatic celiac disease. Gastroenterology152, 787–798.e2 (2017). CASPubMedGoogle Scholar
- Syage, J. A., Murray, J. A., Green, P. H. R. & Khosla, C. Latiglutenase improves symptoms in seropositive celiac disease patients while on a gluten-free diet. Dig. Dis. Sci.62, 2428–2432 (2017). CASPubMedPubMed CentralGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03585478 (2019).
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04243551 (2020).
- Lähdeaho, M.-L. et al. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: a phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol. Hepatol.4, 948–959 (2019). PubMedGoogle Scholar
- Cellier, C. et al. Safety and efficacy of AMG 714 in patients with type 2 refractory coeliac disease: a phase 2a, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Gastroenterol. Hepatol.4, 960–970 (2019). PubMedGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04424927 (2020).
- Waldmann, T. A. The biology of IL-15: implications for cancer therapy and the treatment of autoimmune disorders. J. Investig. Dermatol. Symp. Proc.16, S28–S30 (2013). CASPubMedGoogle Scholar
- Ciszewski, C. et al. Identification of a γc receptor antagonist that prevents reprogramming of human tissue-resident cytotoxic t cells by IL15 and IL21. Gastroenterology158, 625–637.e13 (2020). CASPubMedGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01893775 (2020).
- Yokoyama, S., Perera, P.-Y., Waldmann, T. A., Hiroi, T. & Perera, L. P. Tofacitinib, a janus kinase inhibitor demonstrates efficacy in an IL-15 transgenic mouse model that recapitulates pathologic manifestations of celiac disease. J. Clin. Immunol.33, 586–594 (2013). CASPubMedGoogle Scholar
- EU Clinical Trials Register. ClinicalTrialsRegister.euhttps://www.clinicaltrialsregister.eu/ctr-search/search?query=2018-001678-10 (2018).
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT00540657 (2008).
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04524221 (2020).
- Tato, M. et al. Cathepsin S inhibition combines control of systemic and peripheral pathomechanisms of autoimmune tissue injury. Sci. Rep.7, 2775 (2017). PubMedPubMed CentralGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02679014 (2017).
- Abraham, M. et al. In vitro induction of regulatory T cells by anti-CD3 antibody in humans. J. Autoimmun.30, 21–28 (2008). CASPubMedPubMed CentralGoogle Scholar
- Høydahl, L. S., Frick, R., Sandlie, I. & Løset, G. Å. Targeting the MHC ligandome by use of TCR-like antibodies. Antibodies8, 32 (2019). PubMed CentralGoogle Scholar
- Carballido, J. M. & Santamaria, P. Taming autoimmunity: Translating antigen-specific approaches to induce immune tolerance. J. Exp. Med.216, 247–250 (2019). CASPubMedPubMed CentralGoogle Scholar
- Christophersen, A., Risnes, L. F., Dahal-Koirala, S. & Sollid, L. M. Therapeutic and diagnostic implications of t cell scarring in celiac disease and beyond. Trends Mol. Med.25, 836–852 (2019). CASPubMedGoogle Scholar
- Risnes, L. F. et al. Disease-driving CD4 + T cell clonotypes persist for decades in celiac disease. J. Clin. Invest.128, 2642–2650 (2018). PubMedPubMed CentralGoogle Scholar
- Goel, G. et al. Epitope-specific immunotherapy targeting CD4-positive T cells in coeliac disease: two randomised, double-blind, placebo-controlled phase 1 studies. Lancet. Gastroenterol. Hepatol.2, 479–493 (2017). PubMedPubMed CentralGoogle Scholar
- Daveson, A. J. M. et al. Epitope-specific immunotherapy targeting CD4-positive T cells in celiac disease: safety, pharmacokinetics, and effects on intestinal histology and plasma cytokines with escalating dose regimens of Nexvax2 in a Randomized, double-blind, placebo-controlled. EBioMedicine26, 78–90 (2017). PubMedPubMed CentralGoogle Scholar
- Goel, G. et al. Cytokine release and gastrointestinal symptoms after gluten challenge in celiac disease. Sci. Adv.5, eaaw7756 (2019). CASPubMedPubMed CentralGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03644069 (2019).
- Truitt, K. E. & Anderson, R. P. Editorial: a non-dietary treatment for coeliac disease-two steps forward, one step back? Authors’ reply. Aliment. Pharmacol. Ther.50, 956–957 (2019). PubMedGoogle Scholar
- Freitag, T. L. et al. Gliadin nanoparticles induce immune tolerance to gliadin in mouse models of celiac disease. Gastroenterology158, 1667–1681.e12 (2020). CASPubMedGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03486990 (2020).
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03738475 (2020).
- Kelly, C. et al. CNP-101 prevents gluten challenge-induced immune activation in adults with celiac disease [abstract]. United Eur. Gastroenterol. J. 7, 1421 (2019). Google Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04530123 (2020).
- Clemente-Casares, X. et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature530, 434–440 (2016). CASPubMedGoogle Scholar
- Grimm, A. J., Kontos, S., Diaceri, G., Quaglia-Thermes, X. & Hubbell, J. A. Memory of tolerance and induction of regulatory T cells by erythrocyte-targeted antigens. Sci. Rep.5, 15907 (2015). CASPubMedPubMed CentralGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04248855 (2020).
- Elliot, D. E. & Weinstock, J. V. Where are we on worms? Curr. Opin. Gastroenterol.28, 551–556 (2012). Google Scholar
- Croese, J. et al. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J. Allergy Clin. Immunol.135, 508–516 (2015). CASPubMedGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02754609 (2020).
- Sulic, A.-M., Kurppa, K., Rauhavirta, T., Kaukinen, K. & Lindfors, K. Transglutaminase as a therapeutic target for celiac disease. Expert Opin. Ther. Targets19, 335–348 (2015). CASPubMedGoogle Scholar
- Klöck, C. & Khosla, C. Regulation of the activities of the mammalian transglutaminase family of enzymes. Protein Sci.21, 1781–1791 (2012). PubMedPubMed CentralGoogle Scholar
- Molberg, Ø. et al. T cells from celiac disease lesions recognize gliadin epitopes deamidated in situ by endogenous tissue transglutaminase. Eur. J. Immunol.31, 1317–1323 (2001). CASPubMedGoogle Scholar
- Maiuri, L. et al. Unexpected role of surface transglutaminase type II in celiac disease. Gastroenterology129, 1400–1413 (2005). CASPubMedGoogle Scholar
- Rauhavirta, T. et al. Epithelial transport and deamidation of gliadin peptides: a role for coeliac disease patient immunoglobulin A. Clin. Exp. Immunol.164, 127–136 (2011). CASPubMedPubMed CentralGoogle Scholar
- Lebreton, C. et al. Interactions among secretory immunoglobulin A, CD71, and transglutaminase-2 affect permeability of intestinal epithelial cells to gliadin peptides. Gastroenterology143, 698–707.e4 (2012). CASPubMedGoogle Scholar
- Ventura, M. A. E. et al. Su1161 - The oral transglutaminase 2 (TG2) inhibitor Zed1227 blocks TG2 activity in a mouse model of intestinal inflammation [Abstract]. Gastroenterology154, S-490 (2018). Google Scholar
- EU Clinical Trials Register. ClinicalTrialsRegister.euhttps://www.clinicaltrialsregister.eu/ctr-search/search?query=2017-002241-30 (2017).
- Stamnaes, J., Pinkas, D. M., Fleckenstein, B., Khosla, C. & Sollid, L. M. Redox regulation of transglutaminase 2 activity. J. Biol. Chem.285, 25402–25409 (2010). CASPubMedPubMed CentralGoogle Scholar
- Palanski, B. A. & Khosla, C. Cystamine and disulfiram inhibit human transglutaminase 2 via an oxidative mechanism. Biochemistry57, 3359–3363 (2018). CASPubMedGoogle Scholar
- Gujral, N., Löbenberg, R., Suresh, M. & Sunwoo, H. In-vitro and in-vivo binding activity of chicken egg yolk immunoglobulin Y (IgY) against gliadin in food matrix. J. Agric. Food Chem.60, 3166–3172 (2012). CASPubMedGoogle Scholar
- Sample, D. A. et al. AGY, a novel egg yolk-derived anti-gliadin antibody, is safe for patients with celiac disease. Dig. Dis. Sci.62, 1277–1285 (2017). CASPubMedGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03707730 (2019).
- Stadlmann, V. et al. Novel avian single-chain fragment variable (scFv) targets dietary gluten and related natural grain prolamins, toxic entities of celiac disease. BMC Biotechnol.15, 109 (2015). PubMedPubMed CentralGoogle Scholar
- Liang, L., Pinier, M., Leroux, J.-C. & Subirade, M. Interaction of alpha-gliadin with poly(HEMA-co-SS): structural characterization and biological implication. Biopolymers91, 169–178 (2009). CASPubMedGoogle Scholar
- McCarville, J. L. et al. BL-7010 demonstrates specific binding to gliadin and reduces gluten-associated pathology in a chronic mouse model of gliadin sensitivity. PLoS ONE9, e109972 (2014). PubMedPubMed CentralGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01990885 (2017).
- Caminero, A. et al. Duodenal bacteria from patients with celiac disease and healthy subjects distinctly affect gluten breakdown and immunogenicity. Gastroenterology151, 670–683 (2016). CASPubMedGoogle Scholar
- Hiippala, K. et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients10, 988 (2018). PubMed CentralGoogle Scholar
- Abdel-Gadir, A. et al. Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat. Med.25, 1164–1174 (2019). CASPubMedPubMed CentralGoogle Scholar
- McCarville, J. L. et al. A commensal Bifidobacterium longum strain prevents gluten-related immunopathology in mice through expression of a serine protease inhibitor. Appl. Environ. Microbiol.83, e01323–17 (2017). CASPubMedPubMed CentralGoogle Scholar
- Papista, C. et al. Gluten induces coeliac-like disease in sensitised mice involving IgA, CD71 and transglutaminase 2 interactions that are prevented by probiotics. Lab. Invest.92, 625–635 (2012). CASPubMedGoogle Scholar
- Huibregtse, I. L. et al. Induction of antigen-specific tolerance by oral administration of Lactococcus lactis delivered immunodominant DQ8-restricted gliadin peptide in sensitized nonobese diabetic Abo Dq8 transgenic mice. J. Immunol.183, 2390–2396 (2009). CASPubMedGoogle Scholar
- Olivares, M., Castillejo, G., Varea, V. & Sanz, Y. Double-blind, randomised, placebo-controlled intervention trial to evaluate the effects of Bifidobacterium longum CECT 7347 in children with newly diagnosed coeliac disease. Br. J. Nutr.112, 30–40 (2014). CASPubMedGoogle Scholar
- Quagliariello, A. et al. Effect of Bifidobacterium breve on the intestinal microbiota of coeliac children on a gluten free diet: a pilot study. Nutrients8, 660 (2016). PubMed CentralGoogle Scholar
- Klemenak, M., Dolinšek, J., Langerholc, T., Di Gioia, D. & Mičetić-Turk, D. Administration of Bifidobacterium breve Decreases the production of TNF-α in children with celiac disease. Dig. Dis. Sci.60, 3386–3392 (2015). CASPubMedGoogle Scholar
- Primec, M. et al. Clinical intervention using Bifidobacterium strains in celiac disease children reveals novel microbial modulators of TNF-α and short-chain fatty acids. Clin. Nutr.38, 1373–1381 (2019). CASPubMedGoogle Scholar
- Harnett, J., Myers, S. P. & Rolfe, M. Probiotics and the microbiome in celiac disease: a randomised controlled trial. Evid. Based Complement. Alternat. Med.2016, 9048574 (2016). PubMedPubMed CentralGoogle Scholar
- Francavilla, R. et al. Clinical and microbiological effect of a multispecies probiotic supplementation in celiac patients with persistent IBS-type symptoms: a randomized, double-blind, placebo-controlled, multicenter trial. J. Clin. Gastroenterol.53, e117–e125 (2019). CASPubMedPubMed CentralGoogle Scholar
- Smecuol, E. et al. Exploratory, randomized, double-blind, placebo-controlled study on the effects of Bifidobacterium infantis natren life start strain super strain in active celiac disease. J. Clin. Gastroenterol.47, 139–147 (2013). PubMedGoogle Scholar
- Pinto-Sánchez, M. I. et al. Bifidobacterium infantis NLS super strain reduces the expression of α-defensin-5, a marker of innate immunity, in the mucosa of active celiac disease patients. J. Clin. Gastroenterol.51, 814–817 (2017). PubMedGoogle Scholar
- Vriezinga, S. L. et al. Randomized feeding intervention in infants at high risk for celiac disease. N. Engl. J. Med.371, 1304–1315 (2014). CASPubMedGoogle Scholar
- Lionetti, E. et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N. Engl. J. Med.371, 1295–1303 (2014). PubMedGoogle Scholar
- Håkansson, Å. et al. Effects of Lactobacillus plantarum and Lactobacillus paracasei on the peripheral immune response in children with celiac disease autoimmunity: a randomized, double-blind, placebo-controlled clinical trial. Nutrients11, 1925 (2019). PubMed CentralGoogle Scholar
- Uusitalo, U. et al. Early probiotic supplementation and the risk of celiac disease in children at genetic risk. Nutrients11, 1790 (2019). CASPubMed CentralGoogle Scholar
- US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03562221 (2020).
Acknowledgements
The authors thank the Academy of Finland and the Sigrid Juselius Foundation (K.L.), Emil Aaltonen foundation and the Finnish-Norwegian Medical Foundation (L.K.), the National Health and Medical Research Council of Australia (NHMRC, Investigator Grant APP1176553), and the Mathison Centenary Fellowship, University of Melbourne (J.T.-D.). A.C. holds a Paul Douglas chair in intestinal research.
Author information
Authors and Affiliations
- Tampere Center for Child Health Research, Tampere University and Tampere University Hospital, Tampere, Finland Laura Kivelä
- Children’s Hospital and Pediatric Research Center, University of Helsinki and Helsinki University Hospital, Helsinki, Finland Laura Kivelä
- Department of Medicine, Farncombe Family Digestive Health Research Institute, McMaster University, Hamilton, ON, Canada Alberto Caminero & Maria Ines Pinto-Sanchez
- Harvard Celiac Disease Research Program, Department of Medicine, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA, USA Daniel A. Leffler
- Takeda Pharmaceuticals, Cambridge, MA, USA Daniel A. Leffler
- Immunology Division, The Walter and Eliza Hall Institute, Parkville, and Gastroenterology Department, The Royal Melbourne Hospital, Parkville, Australia Jason A. Tye-Din
- Celiac Disease Research Center, Faculty of Medicine and Health Technology, Tampere University, Tampere, Finland Katri Lindfors
- Laura Kivelä






